Follow us on Google News (click on ☆)
Researchers at the University of California have developed this material, called COF-999. Its CO2 adsorption capacity is exceptional, while also being stable and durable.
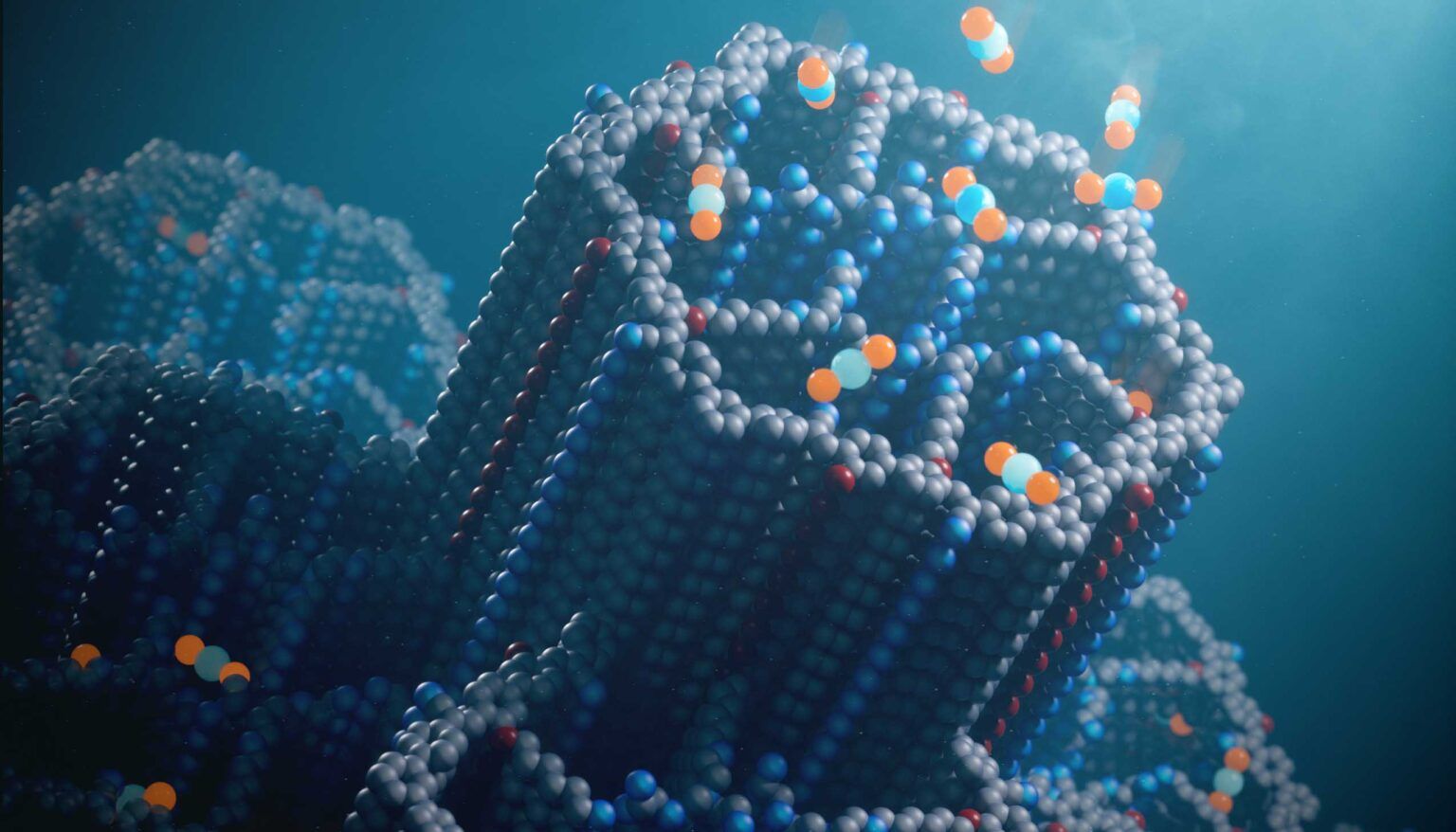
The new porous material for capturing carbon dioxide, called a covalent organic framework (COF), has hexagonal channels lined with polyamines that effectively bind carbon dioxide molecules (blue and orange spheres) at concentrations found in ambient air.
Image Chaoyang Zhao
Carbon dioxide is one of the main drivers of global warming. While reducing emissions is essential, removing this gas directly from the atmosphere has become indispensable.
Traditional materials used for capturing CO2, like MOFs (Metal-Organic Frameworks), hold promise. However, their durability diminishes after several regeneration cycles, limiting their industrial effectiveness.
COF-999, on the other hand, is based on strong covalent bonds (carbon-carbon and carbon-nitrogen), making it more robust. These bonds are known for their high thermal and chemical resistance.
The material works by trapping CO2 in its pores. It can capture about 400 parts per million of CO2 in less than two hours. Moreover, its regeneration requires less energy than similar materials.
Artificial intelligence could further enhance this technology by optimizing the structure of COF-999. This material could eventually be used on a large scale in direct CO2 capture facilities.
Although economic challenges remain, the research team believes this new material could be a major breakthrough in the fight against global warming.
What is a Metal-Organic Framework (MOF)?
A Metal-Organic Framework (MOF) is a porous hybrid material composed of metallic nodes connected by organic linkages. This structure creates internal cavities capable of capturing and storing specific molecules, such as carbon dioxide. MOFs are widely used due to their large internal surface area and chemical flexibility.
MOFs are particularly effective for gas capture thanks to their ability to adsorb molecules in their pores. This means that gases adhere to the internal surface of the material without undergoing major chemical reactions.
What is a Covalent-Organic Framework (COF) and how does it help capture CO2?
Covalent-Organic Frameworks (COF) are porous crystalline structures formed by strong bonds between carbon and nitrogen atoms. These bonds provide them with great chemical and thermal stability, which is crucial for withstanding extreme conditions such as humidity or heat.
Their internal structure is designed to maximize the available surface area to absorb gases. In the case of COF-999, the pores are lined with amines, compounds that interact specifically with CO2, allowing for the efficient capture of this gas in ambient air.
Compared to other capture materials, a COF has the advantage of requiring less energy for regeneration and offering increased durability. This makes it a promising candidate for large-scale industrial applications in the fight against global warming.
The crystalline structure of COFs offers a regular arrangement of microscopic pores, thereby maximizing the surface area available for gas adsorption, such as CO2. This precise architecture allows for more efficient and selective carbon dioxide capture.
By controlling the size and shape of the pores in COFs, it is possible to optimize them for specific applications, such as capturing CO2 from the air. This makes them a customizable and adaptable material, ideal for a variety of industrial conditions.