Follow us on Google News (click on ☆)

Organic molecules, primarily composed of carbon, have well-defined three-dimensional structures. Olefins, for example, are compounds with double bonds between two carbon atoms, generally forming a flat geometry. However, this rule of flatness doesn't always apply, as these new studies show.
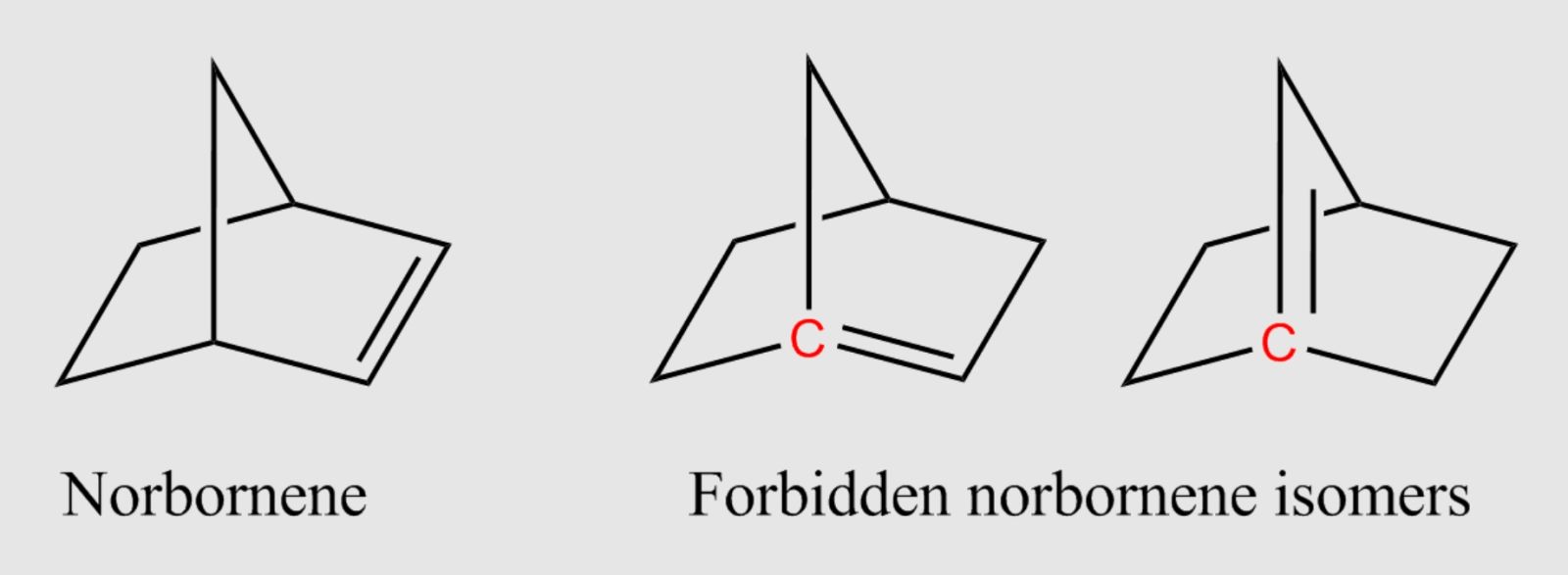
Since 1924, "Bredt's rule" has taught that a carbon-carbon double bond cannot exist at the junction points of a bicyclic molecule, where the angles are too strained. This idea was regarded as immutable, limiting attempts to create new molecules.
Researchers at UCLA have now shown that these constraints aren't absolute. They have successfully synthesized molecules called anti-Bredt olefins, thus contradicting this old paradigm. These structures, although theoretically unstable, can be trapped to create usable compounds in chemistry.
Neil Garg, the chemist behind this breakthrough, criticizes these "immutable rules," which, in his view, stifle the creativity of scientists. He believes that a rigid framework in chemistry can sometimes block crucial innovations, particularly in pharmacology.
In the lab, the team used silylated compounds combined with a fluoride source to induce an elimination reaction. This process generated anti-Bredt olefins. An auxiliary molecule then stabilized these transient structures to extract usable compounds.
Atoms violating Bredt's rule in red.
Wikimedia Image
Their method has drawn particular interest in the pharmaceutical industry. These new molecular geometries could indeed be used to design more effective drugs, according to Garg. The potential of anti-Bredt olefins goes far beyond their mere synthesis: they open the door to unexpected applications.
The research involved several scientists, including students, postdoctoral fellows, and Professor Ken Houk, an expert in computational chemistry. Their collaboration made it possible to understand the mechanisms on a molecular scale and confirm their practical feasibility.
With this discovery, the researchers are calling for a reevaluation of textbooks and for chemistry to be approached with more flexibility. Bredt's principle, once considered a universal truth, could soon be downgraded to a mere guideline.
What is Bredt's rule in organic chemistry?
Bredt's rule, formulated in 1924, concerns the structure of cyclic molecules. It states that a carbon-carbon double bond cannot form at the junction point (called a "bridgehead") in a bicyclic molecule, where the angles around this bond would be too distorted.
This rule is based on the fact that the geometry imposed by the double bond would be unstable. Excessive angular strain would make these molecules difficult to synthesize, and if they did exist, highly reactive or short-lived.
Recent research has shown that it's possible to synthesize compounds violating this rule, called anti-Bredt olefins. These new molecules open up unprecedented avenues in chemistry, particularly in the design of new pharmaceuticals.