Follow us on Google News (click on ☆)
In a study published in Nature, French and English chemists demonstrate how very simple artificial molecular motors are capable of producing such work by catalyzing the conversion of a chiral chemical fuel. This could propel soft robotics and active matter.

Illustration image Pixabay
How can certain molecules perform organized and ordered work in a chaotic environment? The world of molecules immersed in a liquid is indeed governed by a stochastic process, called Brownian motion, which describes the random trajectories of particles.
Forcing one trajectory over another is no easier than moving through a cyclone. Yet, it is in this disordered environment that biological motors, proteins with complex structures, produce perfectly defined mechanical work.
For example, myosin-II is responsible for muscle contraction in most animals. To do this, this enzyme-active protein converts a source of chemical energy (adenosine triphosphate (ATP)) into a reaction product (adenosine diphosphate (ADP)).
This transformation is accompanied by a cascade of perfectly ordered movements that result in the macroscopic contraction of muscle tissue. However, the mechanism behind this conversion of chemical energy into work is still not settled by the scientific community.
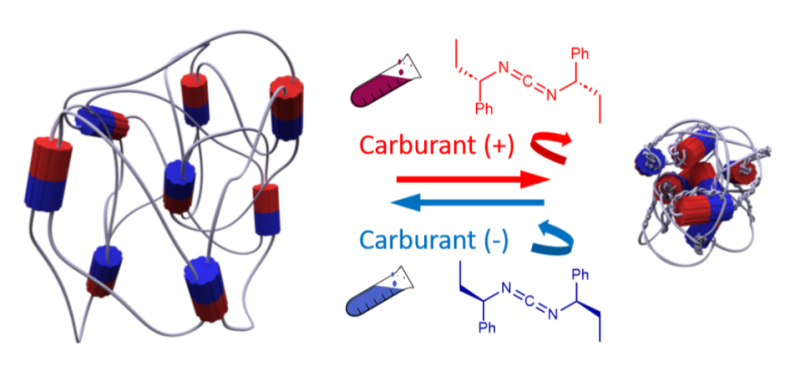
Artificial muscle using rotary molecular motors, activated by a chiral chemical fuel. The direction of rotation of the motors is determined by the enantiomeric excess of the fuel, allowing control of the contraction or extension of the artificial muscle.
© Nicolas Giuseppone
As part of the European collaborative project ITN-ArtMoMa, chemists from the Charles Sadron Institute (CNRS/University of Strasbourg/Institut Universitaire de France) and the University of Manchester have managed to answer this question using a minimal chemical system. They combined small rotary motors (1000 times smaller than myosin-II) with a polymer network to form an active hydrogel.
The molecular motors used are composed of a static part (stator) and a mobile part (rotor). When a chemical fuel is added, the motor catalyzes the transformation of the fuel into a reaction product, which drives the rotation of the rotor through an ordered sequence of conformational changes.
The rotor, chemically linked to the polymer chains, in turn drives the contraction or expansion of the material, depending on the direction of rotation. This preferential direction of rotation transferred to the polymer network is determined by the chirality of the fuel. Depending on the enantiomeric excess of the latter, a macroscopic contraction or extension of the hydrogel is observed.
By measuring changes in the properties of the hydrogel material, the researchers were also able to determine the force generated by the motor and its energy efficiency.
This very simple system demonstrates how a catalyst can convert a source of chemical energy into controlled movements up to the macroscopic scale by playing on the asymmetry of the rate constants of the reactions involved in the catalytic cycle.
This study published in the journal Nature highlights the potential of artificial molecular motors to explain one of the great principles of how living systems work. It could also inspire the design of new active elements in the field of materials science and nanotechnology.
Reference:
Transducing chemical energy through catalysis by an artificial molecular motor
Peng-Lai Wang, Stefan Borsley, Martin J. Power, Alessandro Cavasso, Nicolas Giuseppone & David Leigh.
Nature 2024
https://doi.org/10.1038/s41586-024-08288-x