Follow us on Google News (click on ☆)
A team from the SAMURAI21-NeuLAND collaboration has shown, through the study of a very close nucleus, fluorine-30, that this nucleus is ultimately not magical, and its nucleons are not held more tightly. Instead, a regime of superfluidity takes over, in which neutrons hop effortlessly between entangled orbitals.
Since taking on the challenge of studying oxygen-28, scientists from the SAMURAI collaboration in Japan, which includes physicists from IN2P3, have been encountering one surprise after another. The latest one is quite remarkable.
This nucleus, composed of 8 protons and 20 neutrons, is particularly interesting because, despite its intrinsic characteristics, which should make it a "magic" nucleus—thus more stable than its neighbors—it turns out to be highly unstable: its lifespan does not exceed 10-20 seconds.
The physicists therefore sought to better understand its intrinsic workings to determine whether or not it should be considered magic. And what they found is very interesting. The triumph of instability is due to a narrowing of the orbitals that contain the nucleons, allowing the establishment of a superfluidity regime.
Superfluidity? To better understand this phenomenon, let's dive back into the basics of nuclear physics.
In atomic nuclei, the rule is that when one has just the right number of nucleons to fill its orbitals, it is more stable and is called magical. This effect is further amplified if both protons and neutrons are optimally arranged. In such cases, we speak of a doubly magic nucleus.
Oxygen-28, with its 8 protons and 20 neutrons, belongs to this super-category and should, in theory, have enhanced stability. But another equally unforgiving rule of nuclear physics works in the opposite direction. This time, it is the difference between the number of protons and neutrons that plays a role.
The most stable nuclei have approximately the same number of protons and neutrons. But as this difference increases, the nuclei become less and less stable. With 20 neutrons and 8 protons, oxygen-28 tends strongly toward instability. And in the end, there is no contest: with its extremely short lifespan, it is clear that the rules of instability reign supreme. The only question left was why.
To probe the magicity of oxygen-28, scientists aim to compare the energy required to remove a neutron from oxygen-28 with that needed to do the same for oxygen-29, which has one additional neutron. The idea being that magic oxygen-28 should retain its neutrons more firmly than oxygen-29. However, the ultra-short lifespan of these two isotopes and especially the decay mode of oxygen-29 make this test extremely difficult.
The scientists of the SAMURAI21-NeuLAND collaboration, therefore, proposed bypassing the problem by studying nuclei very close in nucleon numbers, such as fluorine-30 (9 protons, 21 neutrons) and fluorine-29 (9 protons, 20 neutrons). The proximity between the two elements allows the results for fluorine isotopes to be extrapolated to those of oxygen.
The measurements obtained are unequivocal: the energy required to eject a neutron from each of the two fluorine isotopes is comparable, proving that the "magical" and stabilizing configuration at 20 neutrons no longer exists.
"We believe that in this configuration, involving very loosely bound and highly unstable nuclei, the distinct orbitals usually found disappear in favor of a tangle of orbitals between which neutrons can move freely," explains Olivier Sorlin, a researcher at GANIL who participated in the SAMURAI21-NeuLAND study. "This new regime that emerges is probably one of superfluidity, where neutrons pair up and hop from one orbital to another indiscriminately. In this context, the rules of magicity, governed by the filling or not of certain orbitals, no longer apply. This is why oxygen-28 is not magic."
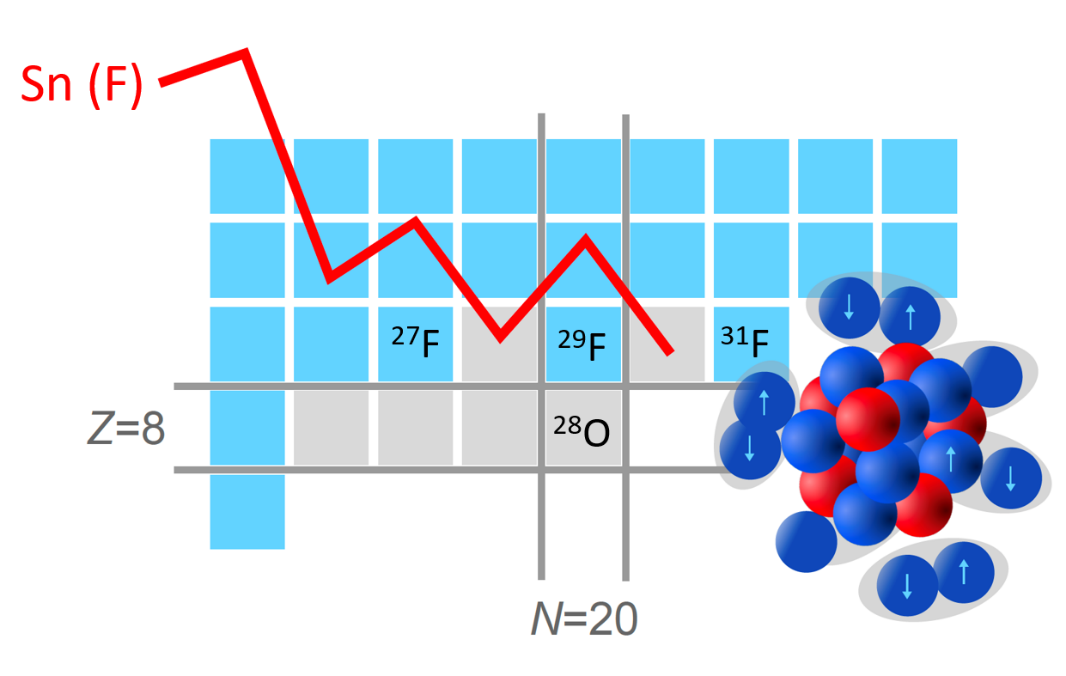
Graph showing the evolution of the neutron separation energy (Sn) for fluorine isotopes. The curve's oscillations are due to the fact that, as neutrons evolve in pairs, this energy is systematically higher in isotopes containing an even number of neutrons than those with an odd number: it requires less energy to remove a lone neutron. Between fluorine-29 and fluorine-30, the oscillations remain constant. This proves that the neutron orbitals are indeed entangled. The presence of the magic number N=20 would have caused a drop in Sn between fluorine-29 and fluorine-30.
But the surprises don't stop there. If this is the first time a superfluidity regime has been observed in exotic nuclei, the scientists have also made another startling discovery.
"Until now, we thought that neutron pairing in a context of superfluidity occurred within the atomic nucleus only over long distances, when the two neutrons in a pair are far apart," continues Olivier Sorlin. "But the theoretical models that reproduce the experimental results for fluorine and oxygen nuclei suggest that the neutron pairs are much closer. If this result were to be confirmed by new, more specialized experiments, planned by the SAMURAI collaboration, it would radically change our understanding of superfluidity."
Clearly, with the unexpected appearance of superfluidity in place of magicity, the SAMURAI collaboration has paved the way for a new, exciting quest.