Nanoplastics, the asbestos of the 21st century? 🔬
Published by Cédric,
Article author: Cédric DEPOND
Source: Nature Communications
Other Languages: FR, DE, ES, PT
Article author: Cédric DEPOND
Source: Nature Communications
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)

Micro- and nanoplastics are contaminating all ecosystems, from Antarctic snow to human blood. A study published in Nature Communications reveals why these materials, despite being resistant, break down into potentially toxic particles. Led by Sanat Kumar's team (Columbia Engineering), this research highlights an intrinsic process in common plastics.
The hidden structure of plastics
Semi-crystalline plastics, which make up the majority of our daily use (accounting for 75 to 80% of our consumption), owe their properties to a unique molecular organization. Their structure resembles thousands of alternating rigid and flexible layers. Think of it as a stack of bricks (rigid crystalline zones) held together by mortar (flexible amorphous zones). This alternating pattern explains their mechanical strength and adaptability, but also their long-term vulnerability.
By observing these materials under an electron microscope, researchers found that the flexible layers gradually deteriorate under the effects of heat, UV rays, or chemical stress. Contrary to popular belief, this degradation occurs even without physical pressure, simply through aging. The weak molecular bonds in the flexible zones are the first to break.
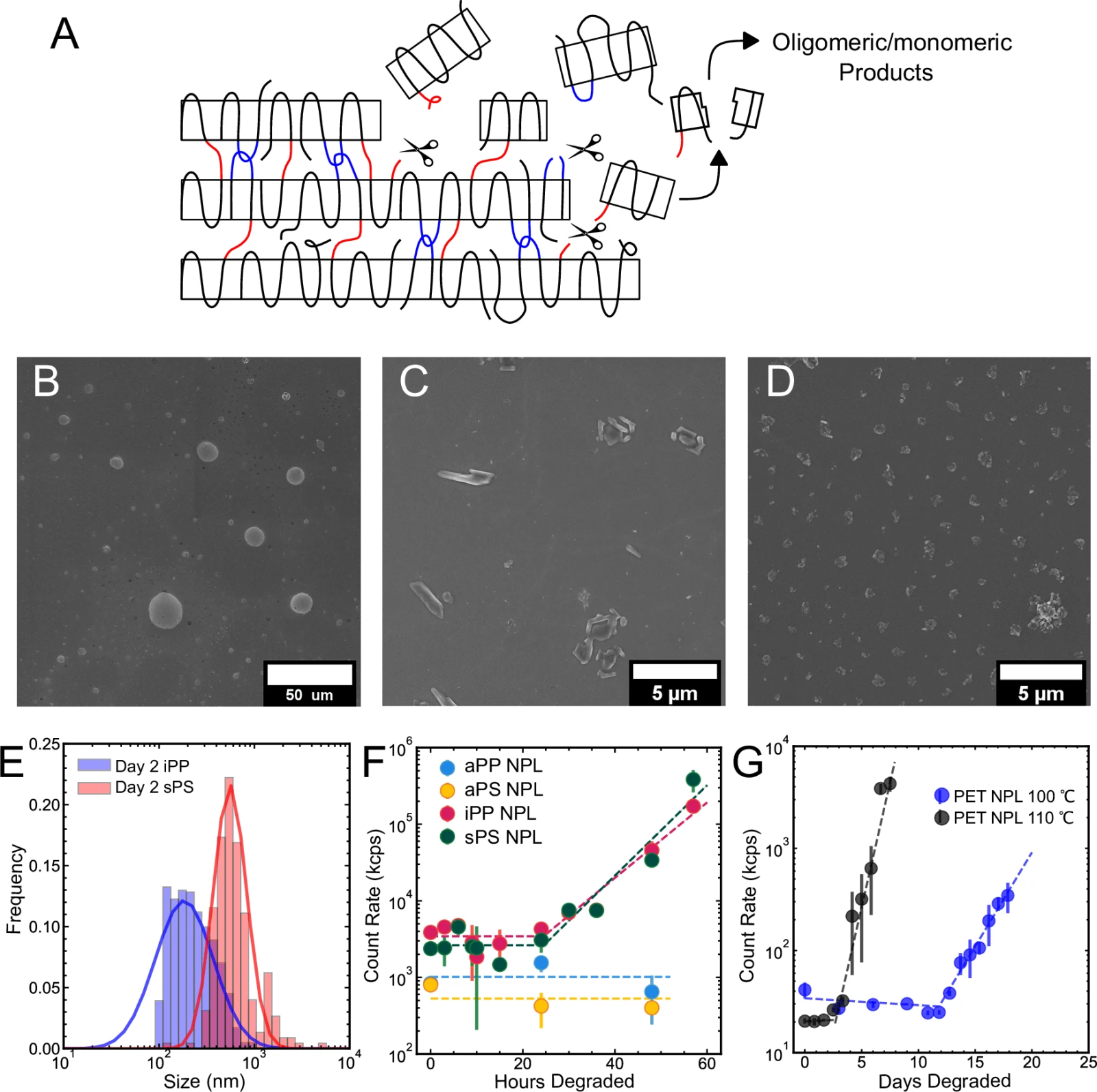
A) Diagram showing chain breakage in plastic.
B) Image of a flexible polypropylene film (aPP) after 2 days.
C) Image of a structured polystyrene film (sPS) with small particle formation.
D) Image of a rigid polypropylene film (iPP) with the same particles.
E) Size of particles formed in sPS and iPP films.
F) Light measurement during the degradation of various plastics (aPP, sPS, iPP, and standard polystyrene).
G) Measurement during PET degradation (plastic used in bottles).
The study particularly reveals a domino effect: when the flexible layers break, they release adjacent crystalline fragments. These fragments, stable and indestructible on a human scale, become the nanoplastics found in the environment. Their nanometric size (0.001 to 0.1 μm) makes them particularly dangerous, as it allows them to cross biological barriers.
Major health implications
The persistence of nanoplastics in the environment poses an unprecedented health challenge. These particles, resistant to degradation, accumulate in soils, oceans, and even the atmosphere, creating constant exposure. Their microscopic size enables them to easily enter the food chain, inevitably ending up in the human body.
Preliminary studies suggest concerning mechanisms of action: once inhaled or ingested, nanoplastics can cross cell walls and disrupt biological functions. Some research indicates they may accumulate in cell nuclei, potentially interfering with DNA. Their elongated shape and durability recall the carcinogenic properties of asbestos, though long-term studies are still needed.
Faced with these risks, the research team emphasizes the urgency of preventive measures. Better polymer design could reduce nanoplastic generation at the source. Meanwhile, developing effective detection and filtration methods becomes crucial to limit human exposure to these invisible yet potentially devastating particles.