Lithium extraction: the end of polluting methods? 🔋
Published by Cédric,
Article author: Cédric DEPOND
Source: Nature Communications
Other Languages: FR, DE, ES, PT
Article author: Cédric DEPOND
Source: Nature Communications
Other Languages: FR, DE, ES, PT
Follow us on Google News (click on ☆)

This innovation, developed by scientists at Pennsylvania State University, relies on an electrochemical process that avoids extreme temperatures and harsh chemicals. By combining an electric current and hydrogen peroxide, they manage to extract lithium directly from spodumene ore, with efficiency comparable to traditional methods.
The limitations of current methods
Today, 70% of lithium is extracted from brines, a slow process that dries up soils and destroys ecosystems. The other method, based on ore processing, requires temperatures reaching 1,100°C (2,012°F) and concentrated acids, leading to high energy consumption and environmental risks.
These techniques, although effective, are incompatible with sustainability goals. The growing demand for lithium, essential for the energy transition, requires more environmentally friendly and economically viable solutions.
The principle of the electrochemical method
The new approach uses an electric field to release lithium ions from spodumene, without requiring high temperatures or harsh chemicals. Hydrogen peroxide acts as a catalyst, facilitating the reaction and improving the efficiency of the process.
This method achieves an extraction rate of 92.2%, comparable to traditional techniques, while significantly reducing energy costs and CO₂ emissions. It paves the way for more sustainable and faster lithium production.
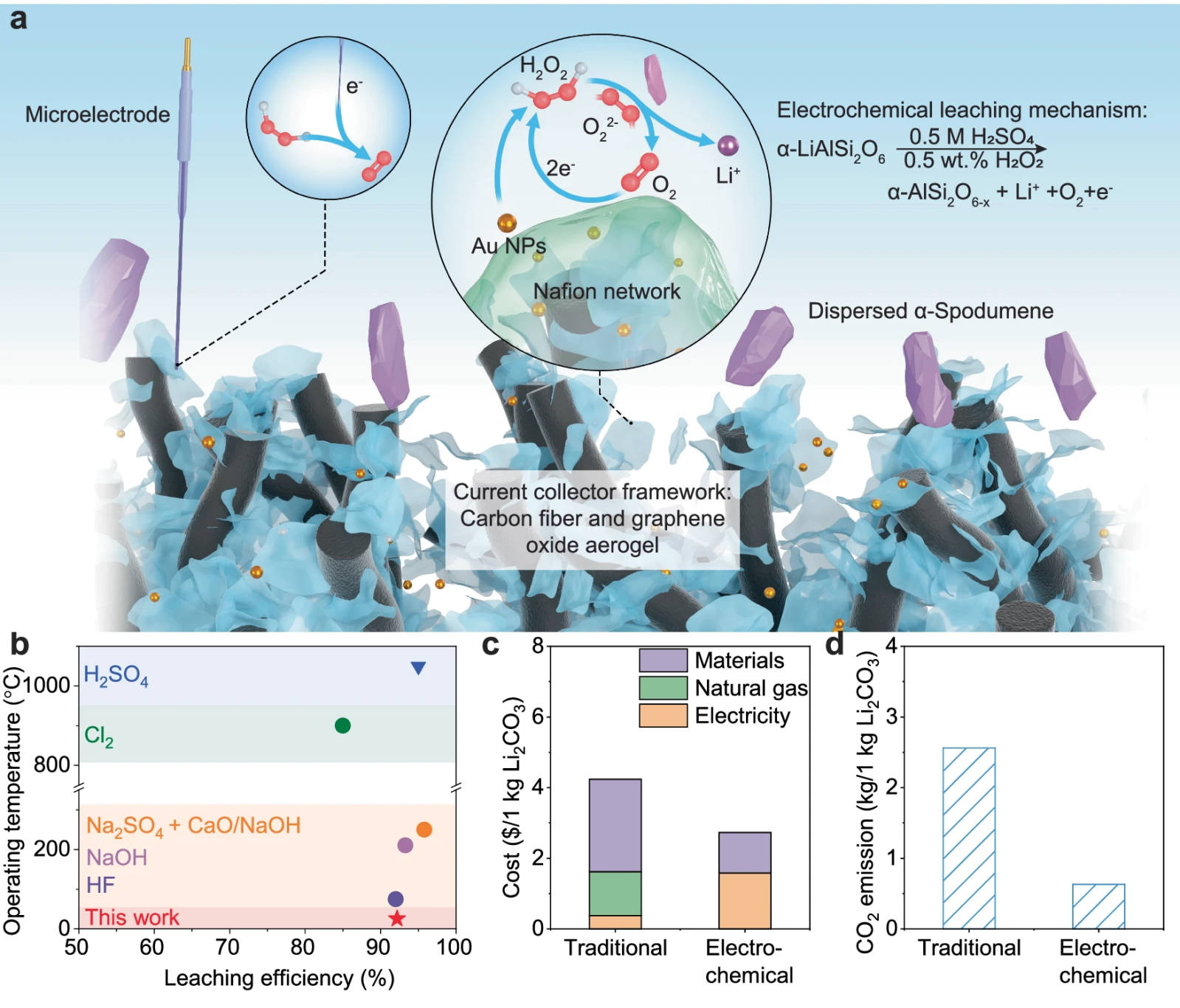
a) Diagram of the operation of the modified current collector, optimizing surface area and conductivity to improve the reaction.
b) Comparison of temperature and efficiency of leaching methods, including the influence of chemical agents and pressure.
c) Cost analysis of traditional and electrochemical methods, excluding labor and infrastructure.
d) CO₂ emissions assessment of both methods, assuming electricity from fossil sources.
Economic and environmental benefits
By eliminating the need to heat ores to extreme temperatures, this method reduces operating costs by 35.6%. It also decreases CO₂ emissions by 75.3%, thanks to optimized energy consumption and the absence of natural gas in the process.
These savings could be passed on to battery prices, making electric vehicles more accessible. Moreover, this technology would reduce dependence on dominant countries in refined lithium production, such as China.
Future prospects
Researchers are now working to improve the process to enable direct recovery of lithium in solid form, ready for industrial use. This step is essential to make the method viable on a large scale.
If these efforts succeed, this innovation could transform the lithium industry, aligning production with environmental goals. It represents a major advance for the energy transition and the fight against climate change.
To go further: What is electrochemistry applied to lithium extraction?
Electrochemistry is a branch of chemistry that studies the reactions between electricity and chemical substances. In the context of lithium extraction, it allows the release of lithium ions from ores without resorting to extreme temperatures or harsh chemicals.
This method relies on applying an electric current to an ore, such as spodumene, immersed in a solution containing a catalyst, such as hydrogen peroxide. The current facilitates the separation of lithium ions, making the process more efficient and less energy-intensive.
Unlike traditional techniques, electrochemistry does not require heating ores to temperatures exceeding 1,000°C (1,832°F). It thus reduces energy consumption and CO₂ emissions, while minimizing chemical waste.
This approach opens new perspectives for the mining industry, aligning resource extraction with environmental respect. It could also apply to other critical metals essential for the energy transition.
What is spodumene and why is it important for lithium?
Spodumene is a lithium-rich ore, primarily composed of aluminum and lithium silicate. It is one of the most important sources of lithium, used in the manufacture of batteries for electric vehicles and electronic devices.
This ore is found in hard rocks, often extracted in open-pit mines. Traditionally, its processing requires extreme temperatures and strong acids to release lithium, resulting in high costs and significant environmental impact.
Spodumene is therefore a key resource to meet the growing demand for lithium, and its sustainable exploitation is essential to support the energy transition while preserving the environment.