Follow us on Google News (click on ☆)

Scientists from the University of Geneva (UNIGE), in collaboration with the Structural Biology Institute of Grenoble (IBS) and the University of Fribourg (UNIFR), have employed cryo-electron microscopy to observe how lipids and proteins in the plasma membrane interact and respond to mechanical stress.
Their research shows that, depending on conditions, small membrane regions can stabilize different lipids to trigger specific cellular responses. These findings, published in the journal Nature, confirm the existence of well-organized lipid domains and begin to uncover their role in cell survival.
Cells are surrounded by a membrane—the plasma membrane—that acts as a physical barrier but must remain flexible. These properties are conferred by the membrane's constituent elements, lipids and proteins, whose molecular organization varies according to the external environment. This dynamism is essential for membrane function, but it must be finely tuned so that the membrane does not become too tense or too slack.
The way cells perceive changes in the biophysical properties of the plasma membrane likely involves small regions of the membrane—known as microdomains—that have specific content and organization of lipids and proteins.
High-resolution cryo-electron microscopy
The team led by Robbie Loewith, a full professor in the Department of Molecular and Cellular Biology at the Faculty of Sciences of UNIGE, is interested in how the components of the plasma membrane interact to maintain optimal biophysical properties for cell growth and survival.
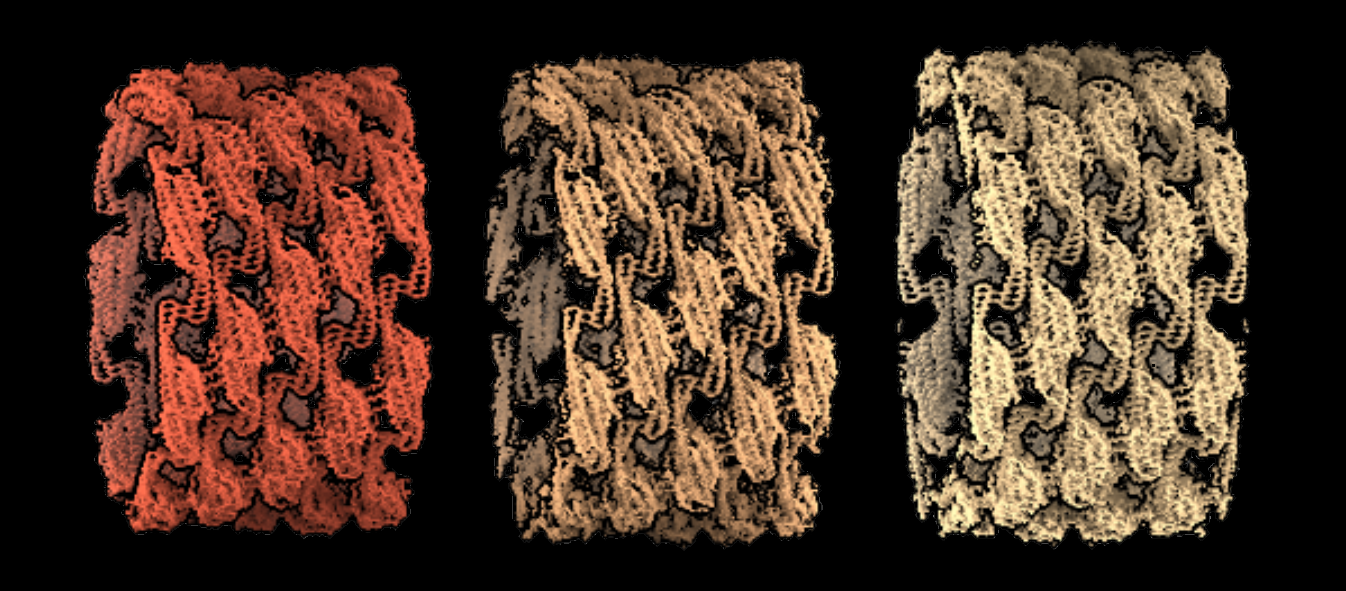
Three-helix structure of the eisosome, a stretch-sensitive membrane microdomain of baker's yeast, resolved by high-resolution cryo-electron microscopy.
© Jennifer Kefauver, Ambroise Desfosses, Luoming Zou, Robbie Loewith
"Until now, available techniques did not allow us to study lipids within their natural environment inside membranes. Thanks to the Dubochet Center for Imaging (DCI) of the universities of Geneva, Lausanne, Bern, and EPFL, we overcame this challenge using cryo-electron microscopy," explains Robbie Loewith. This technique allows for freezing samples at -328°F (-200°C) to capture membranes in their native state, which can then be observed under an electron microscope.
The scientists used baker's yeast (Saccharomyces cerevisiae), a model organism in many research laboratories because it is very easy to cultivate and genetically manipulate. Furthermore, most of its fundamental cellular processes are similar to those of higher organisms. This study focused on eisosomes, which are specific membrane microdomains organized around a network of proteins. These eisosomes might be capable of sequestering or releasing proteins and lipids to help cells withstand membrane damage and/or signal it, according to previously unknown processes.
"For the first time, we managed to purify and observe eisosomes containing lipids from the plasma membrane in their native state. This is a significant advance in better understanding their function," explains Markku Hakala, a postdoctoral researcher in the Department of Biochemistry at the Faculty of Sciences of UNIGE and co-author of the study.
Converting a mechanical signal into a chemical signal
Thanks to cryo-electron microscopy, the scientists observed that the lipid organization of these microdomains is altered in response to mechanical stress. "We discovered that when the protein network of the eisosome is stretched, for example, due to mechanical pressure, the complex arrangement of lipids within the microdomains is modified. This reorganization of lipids likely allows the release of sequestered signaling molecules to trigger stress adaptation mechanisms. Our study reveals a molecular mechanism by which mechanical stress can be converted into biochemical signaling through protein-lipid interactions with unprecedented precision," explains Jennifer Kefauver, a post-doctoral researcher in the Department of Molecular and Cellular Biology and the lead author of the study.
This research opens many avenues for exploring the crucial role of membrane compartmentalization—that is, the movement of proteins and lipids within membranes to form sub-compartments, the microdomains. This mechanism enables cells to perform specialized biochemical functions, particularly the activation of cellular communication pathways in response to various stresses that cells may encounter.