Follow us on Google News (click on ☆)
This discovery challenges a theoretical model established for over 60 years and opens new perspectives for understanding neurodevelopmental disorders related to synaptic deficits.
The connections between neurons, called synapses, are the essential functional units of the brain. There are different types, allowing a wide variety of neurons to be connected in complex yet precise circuits regulating all brain functions.
In 1963, Roger Sperry proposed a fundamental hypothesis, known as "chemoaffinity": according to this theory, each type of synapse would be defined by a unique combination of molecules, determined during the genesis of neurons. While decades of research have identified many adhesion molecules involved in the formation and maintenance of different types of synapses, the existence of molecular combinations specific to each type of connection, as well as their instructive role during development, had never been demonstrated until now.
A new vision of the development of synapse diversity
In a study published in Nature Neuroscience, scientists revealed an unexpected mechanism of synapse development through the study of the cerebellum, a brain structure responsible for motor coordination and involved in many cognitive processes.
In this structure, Purkinje cells receive two types of excitatory synapses: climbing fiber synapses and parallel fiber synapses. While these two types of connections initially form on the same territory of the Purkinje cell, they eventually occupy distinct territories and acquire very different properties at maturity. By combining transcriptomic approaches, bioinformatics, and genetic manipulations in mice, scientists first demonstrated that distinct combinations of molecules characterize these two types of synapses in the mature network.
However, contrary to expectations, the study reveals that these combinations are not predefined but evolve sequentially during the development and maturation of the network.
Development rules specific to each type of synapse
Surprisingly, the results also show that climbing fiber and parallel fiber synapses initially use a common presynaptic molecule to establish their connections with Purkinje cells. Subsequently, climbing fiber neurons gradually develop a specific molecular signature, releasing new adhesion molecules at their synapses, while parallel fiber connections retain their initial identity.
This discovery explains why these two types of fibers first share the same territory on Purkinje cells before separating into distinct territories, a segregation that directly results from the divergence of their molecular codes. Remarkably, these codes are partly determined by secreted molecules, rather than by "classical" adhesion molecules.
The scientists ultimately demonstrated that the electrical activity of climbing fiber neurons regulates the molecular maturation of their own synapses, suggesting that external factors, such as sensorimotor experience, could specifically modulate certain types of neural connections during this early period of development.
Towards a better understanding of neurodevelopmental disorders
This new model of sequential development of neural connections could apply to other regions of the brain where various neurons connect to well-defined territories of their target, just as in the cerebellum. This hypothesis is reinforced by the presence of the molecules identified at climbing and parallel synapses throughout the brain, where they could play a similar role in encoding synapse identity.
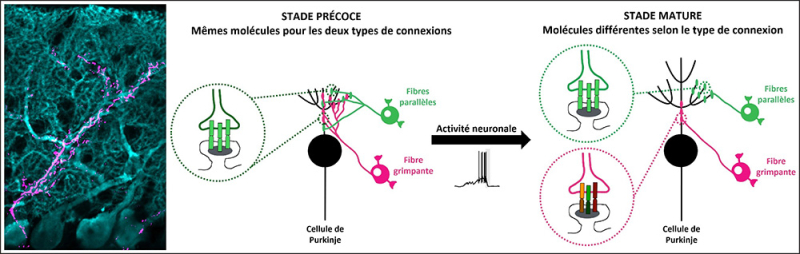
Left: Microscopy image showing Purkinje cells (cyan) as well as a climbing fiber and its synapses (magenta).
Right: This illustration represents the evolution during postnatal development in mice of the two types of excitatory connections on Purkinje cells (black): climbing fiber synapses (pink) and parallel fiber synapses (green). Initially, these two types of synapses share the same molecular identity and the same territory on the growing Purkinje cell, then their territories diverge when at maturity climbing fiber synapses acquire their own identity. Neuronal activity partly modulates the molecular identity and territory of climbing fiber synapses.
© Maëla Paul, CIRB
This fundamental discovery opens new perspectives for understanding the complexity of brain circuit formation and the origin of certain symptoms present in brain development disorders such as autism spectrum disorders or schizophrenia.
Reference:
Paul MA*, Sigoillot SM* et al., Stepwise molecular specification of excitatory synapse diversity onto cerebellar Purkinje cells. Nature Neuroscience. Published online December 10, 2024. https://doi.org/10.1038/s41593-024-01826-w
* Equal contribution.